Welcome everyone to the first of many presentations that the Computer System Validation LCM team has focused on for this page. Today, we'll be discussing 21 CFR Part 11 regulations and how they pertain to us at Illumina Consumables Manufacturing. At a high level, very high level, and yes, that is a spaceship. 21 CFR Part 11, as defined by the FDA, are regulations that set forth the criteria under which the agency considers electronic records, electronic signatures, and handwritten signatures executed to the electronic record to be trustworthy, reliable, and generally equivalent to paper records and handwritten signatures executed on paper. Part 11 regulations are similar to Part 20 regulations, which are quality system regulations for medical device manufacturing. These are regulations that Illumina has complied with in order to continue manufacturing medical devices. Part 11 regulations can be categorized into three key elements: electronic records, electronic signatures, and audit trails. Now, let's dive a little deeper into each of these subparts. Electronic records are a combination of text, graphics, data, pictures, or audio represented in a digital format equivalent to a paper record. These records are created, modified, archived, and retrieved by a computer system. And I'm going to learn how to spell "records" immediately after this video. Electronic signatures can be defined as a combination of symbols representing the username and password, serving as a legally binding equivalent of an individual's handwritten signature. As you can see here, we're utilizing the combination of John Doe's username and password as an electronic signature in our electronic batch record, which is equivalent to John Doe signing off on a paper batch record. Now, let's talk about audit trails. The main purpose of audit trails is to ensure the integrity of the electronic record. Various agency regulations, as well as guidance documents, provide...
Award-winning PDF software





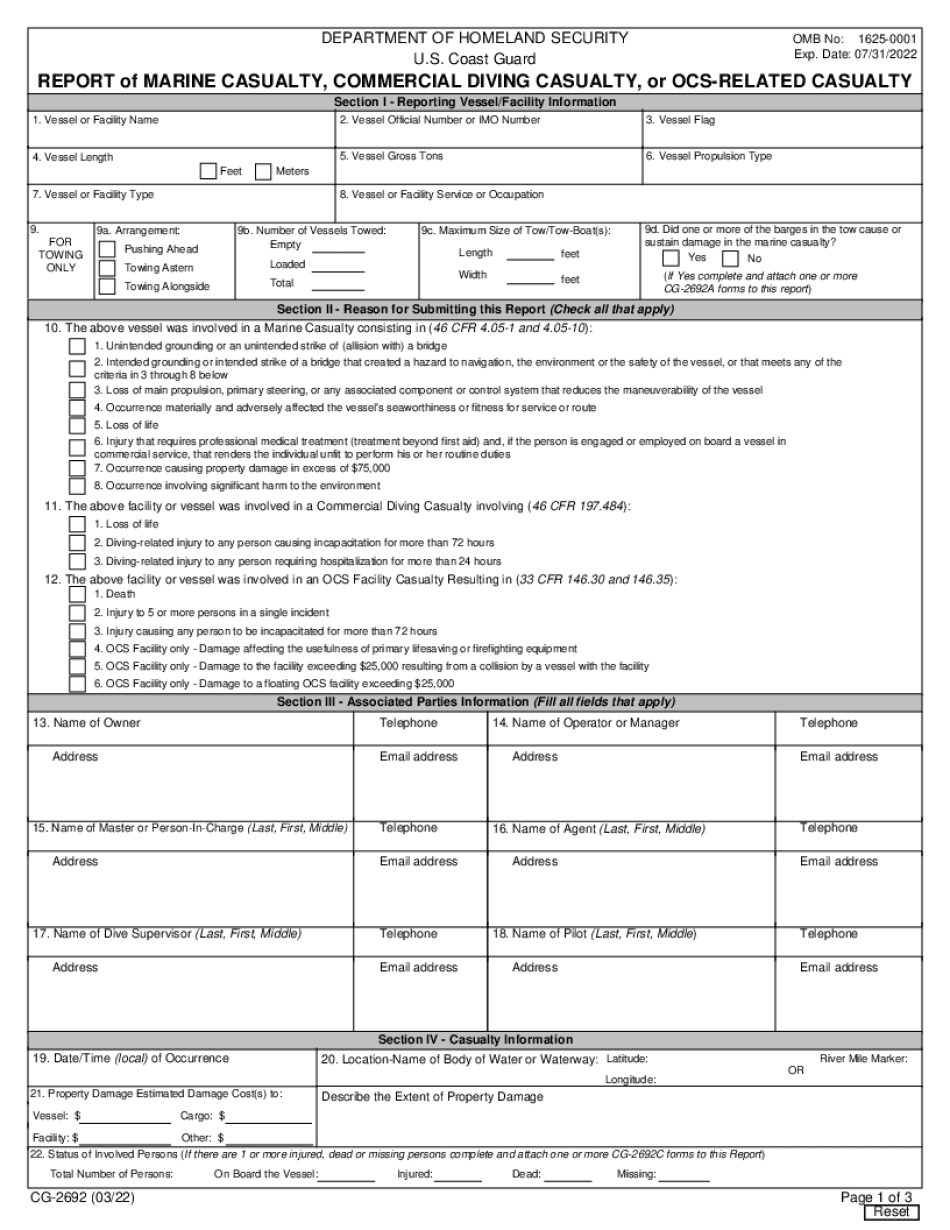
Cfr Marine Form: What You Should Know
Ii) With an application for the issuance of a certificate of listing (which applies only to foreign vessels). (b) Whenever the owner or other person in charge of a vessel for which certificates of listing are required by this chapter, or any other person designated by the owner or other person in charge, commits any act specified, the certificate of listing shall be revoked. When the certificate of listing is revoked, the owner or other person in charge of the vessel may not take the vessel out of the United States without authority from the issuing agency. Any vessel required to have a certificate of listing issued under this chapter shall be declared to be a floating deadweight tonnage vessel unless the vessel: Except by order of an issuing agency, a floating deadweight tonnage vessel may not be taken out of the United States, except under an emergency authorization, without authority from the issuing agency. The authority to take the vessel out of the United States, except under an emergency authorization or under paragraph (b)(1) or (b)(2) of this section, may not be based on a weight or tonnage figure provided for any country, or on any other figure, in a list approved for use by the International Chamber of Shipping or on a statement submitted to, or entered in, the list of countries of the International Chamber of Shipping, any other body recognized as a competent authority to interpret the convention that establishes common rules and procedures with respect to the prevention and control of collision or collision damage, provided that such statement is based on reasonable assumptions as to the condition and condition of vessels in the water. This paragraph shall not apply in cases where-- (i) the vessel is going out of the United States on an errand or for a temporary reason; or (ii) the vessel is a foreign ship registered in a country or territory described in paragraph (b)(1) of this section, except that nothing in this paragraph shall interfere with the validity of documents required from an issuing authority under section 1201 of this title. Section 15.11(a)(1) -- Shipping of foreign merchant vessels overstating weight. SECTION 10.19(d) -- Penalties for violating minimum weight requirements.
online solutions help you to manage your record administration along with raise the efficiency of the workflows. Stick to the fast guide to do CG-2692 Form, steer clear of blunders along with furnish it in a timely manner:
How to complete any CG-2692 Form online: - On the site with all the document, click on Begin immediately along with complete for the editor.
- Use your indications to submit established track record areas.
- Add your own info and speak to data.
- Make sure that you enter correct details and numbers throughout suitable areas.
- Very carefully confirm the content of the form as well as grammar along with punctuational.
- Navigate to Support area when you have questions or perhaps handle our assistance team.
- Place an electronic digital unique in your CG-2692 Form by using Sign Device.
- After the form is fully gone, media Completed.
- Deliver the particular prepared document by way of electronic mail or facsimile, art print it out or perhaps reduce the gadget.
PDF editor permits you to help make changes to your CG-2692 Form from the internet connected gadget, personalize it based on your requirements, indicator this in electronic format and also disperse differently.
Video instructions and help with filling out and completing Cfr marine